


healthcare professional
In this double-blind, time-to-event trial, 143 adults were randomly assigned in a 2:1 ratio to receive either intravenous SOLIRIS® (900 mg weekly dose for the first four doses starting on day 1, followed by 1200 mg every 2 weeks starting at week 4) or matched placebo. The continued use of stable-dose immunosuppressive therapy was permitted.2
The primary endpoint was the first adjudicated relapse. Secondary outcomes included the adjudicated annualised relapse rate, quality-of-life measures and the score on the Expanded Disability Status Scale (EDSS), which ranges from 0 (no disability) to 10 (death).2* Patients were vaccinated against Neisseria meningitidis before receiving a trial agent unless a previous vaccination provided adequate coverage; a 14-day course of antibiotics was administered if there were fewer than 14 days between vaccination and starting trial treatment.
† Randomisation was stratified across sites by Expanded Disability Status Scale (EDSS) score on day 1 (≤ 2.0 and 2.5–7.0) and use of supportive immunosuppressive therapy (IST; no previous IST, unchanged IST or changed IST). IST was considered to be unchanged if no therapy had been started or discontinued after the last relapse before screening, although doses may have changed; patients who had previously received only glucocorticoid therapy were considered to have received no previous IST. Block sizes of three were used within strata.
‡ Patients received 900 mg of eculizumab weekly for the first four doses in the trial; the following week patients started the maintenance regimen, which was 1200 mg every 2 weeks.
§ IST received at screening for the PREVENT core trial was continued unchanged unless treating physicians determined that a relapse had occurred or there was a compelling medical need for adjustment, whereas IST in use in the PREVENT extension trial may be changed at the discretion of the treating physician. Medications not permitted in either trial: rituximab or other biological agents, mitoxantrone, and immunomodulatory therapies (including interferon beta-1a and -1b, and glatiramer acetate); and intravenous immune globulin and plasma exchange for relapse prevention. The patients who completed the trial (owing to a physician-determined relapse or because the trial ended) could choose to enter an extension trial and receive open label treatment with eculizumab. The blind induction period in the extension trial was included to protect blinding in the core trial. The visit interval between studies was 14±2 days from the last administration of trial treatment in the core trial to ensure that there would be no interruptions in trial treatment. Patients not entering the extension trial attended a follow-up visit 8 weeks after the last dose of trial treatment.


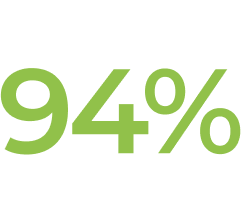
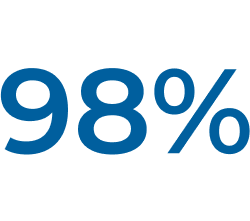
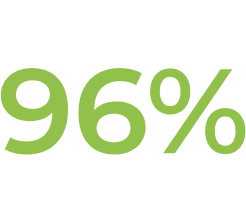

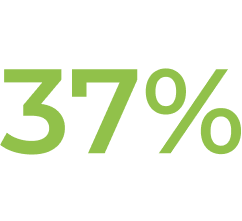
† In Study ECU-NMO-302, physicians had the option to adjust background immunosuppressant therapies. In this setting, the most common change in immunosuppressant therapy was decreased immunosuppressant therapy dose, which occurred in 21.0% of patients. Further, 15.1% of patients stopped an existing immunosuppressant therapy (IST).1
AQP4, aquaporin-4; CI, confidence interval; EDSS, Expanded Disability Status Scale; IST, immunosuppressant therapy; MAC, membrane attack complex; NMOSD, neuromyelitis optica spectrum disorder.