

Metabolic conditions
(weight gain, hyperglycaemia, diabetes)
Osteoporosis
Neuropsychiatric issues
Ophthalmologic conditions
Cardiovascular issues
(hypertension and arrhythmias) especially if pre-existing
Gastrointestinal issues
Electrolyte imbalances
Increased risk of infection
Myopathy
Consider exploring options that may allow you to reduce or eliminate corticosteroids3,6

healthcare professional
CHAMPION-MG OLE was the long-term extension trial following the 26th week of CHAMPION-MG. 161 participants received open-label ULTOMIRIS® following Week 26 and were observed through Week 60.8
LIMITATION: Results and clinical outcomes should be interpreted with caution since the study was designed to evaluate safety and lacked a control group.8,13- At baseline, approximately 90% of patients were taking an immunosuppressive therapy (IST) across both treatments13
- At the time of ULTOMIRIS® initiation, in the ULTOMIRIS® arm, approximately 65% of patients were receiving corticosteroids (prednisone)13
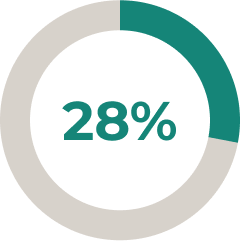
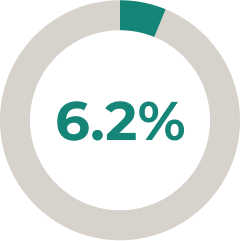
- 5.6% (9/161) of patients increased their corticosteroid use and 2.5% (4/161) of patients initiated corticosteroid use8
- Percentages are based on all patients in the OLE, not just those on steroids8
Corticosteroid use will continue to be observed throughout the ongoing CHAMPION-MG OLE.8
CHAMPION-MG STUDY LIMITATION: Results and clinical outcomes should be interpreted with caution since the study was designed to evaluate safety and lacked a control group.8,13ULTOMIRIS® is the first and only long-acting C5 inhibitor that demonstrates the potential for steroid reduction1,8
* For patients who had received ULTOMIRIS® during the RCP and subsequently entered the OLE (N = 78), the mean MG-ADL total score at RCP baseline was 9.2.8
At Week 60, compared with RCP baseline, improvements of ≥ 3 points on the MG-ADL scale were experienced by 76.4% (n = 42) of patients.8
CHAMPION-MG OLE STUDY LIMITATION: Results should be interpreted with caution since the study was designed to evaluate safety and lacked a control group.8
* In a prespecified interim ULTOMIRIS® treatment analysis (N = 161), 28% of patients reduced and 6.2% of patients stopped corticosteroid use after 34 weeks of the open-label extension (OLE) period (45 and 10 patients, respectively).8
† For patients treated with ULTOMIRIS® during the RCP who subsequently entered the OLE (N = 78), from RCP baseline to Week 60 the LS mean (95% CI) change in MG-ADL total score was -4.0 (-4.8, -3.1). The mean MG-ADL total score for this cohort at RCP baseline was 9.2.8ULTOMIRIS® is the first and only long-acting, targeted C5 inhibitor to report on corticosteroid use. Some patients treated with ULTOMIRIS® may be able to reduce their regular corticosteroid dosage1,8,15,16

To reduce this risk of infection, all patients must be vaccinated against meningococcal infections at least two weeks prior to initiating ULTOMIRIS® unless the risk of delaying ULTOMIRIS® therapy outweighs the risk of developing a meningococcal infection. Patients who initiate ULTOMIRIS® treatment less than 2 weeks after receiving a meningococcal vaccine, must receive treatment with appropriate prophylactic antibiotics until 2 weeks after vaccination. Vaccines against serogroups A, C, Y, W135 and B where available, are recommended in preventing the commonly pathogenic meningococcal serogroups. Patients must be vaccinated or revaccinated according to current national guidelines for vaccination use. If the patient is being switched from SOLIRIS® treatment, physicians should verify that meningococcal vaccination is current according to national guidelines for vaccination use.1
Please consult the Summary of Product Characteristics prior to prescribing.Please report any adverse events via your national reporting system. Adverse events can also be reported to Alexion Pharmaceuticals by the following link: https://contactazmedical.astrazeneca.com/
