


healthcare professional
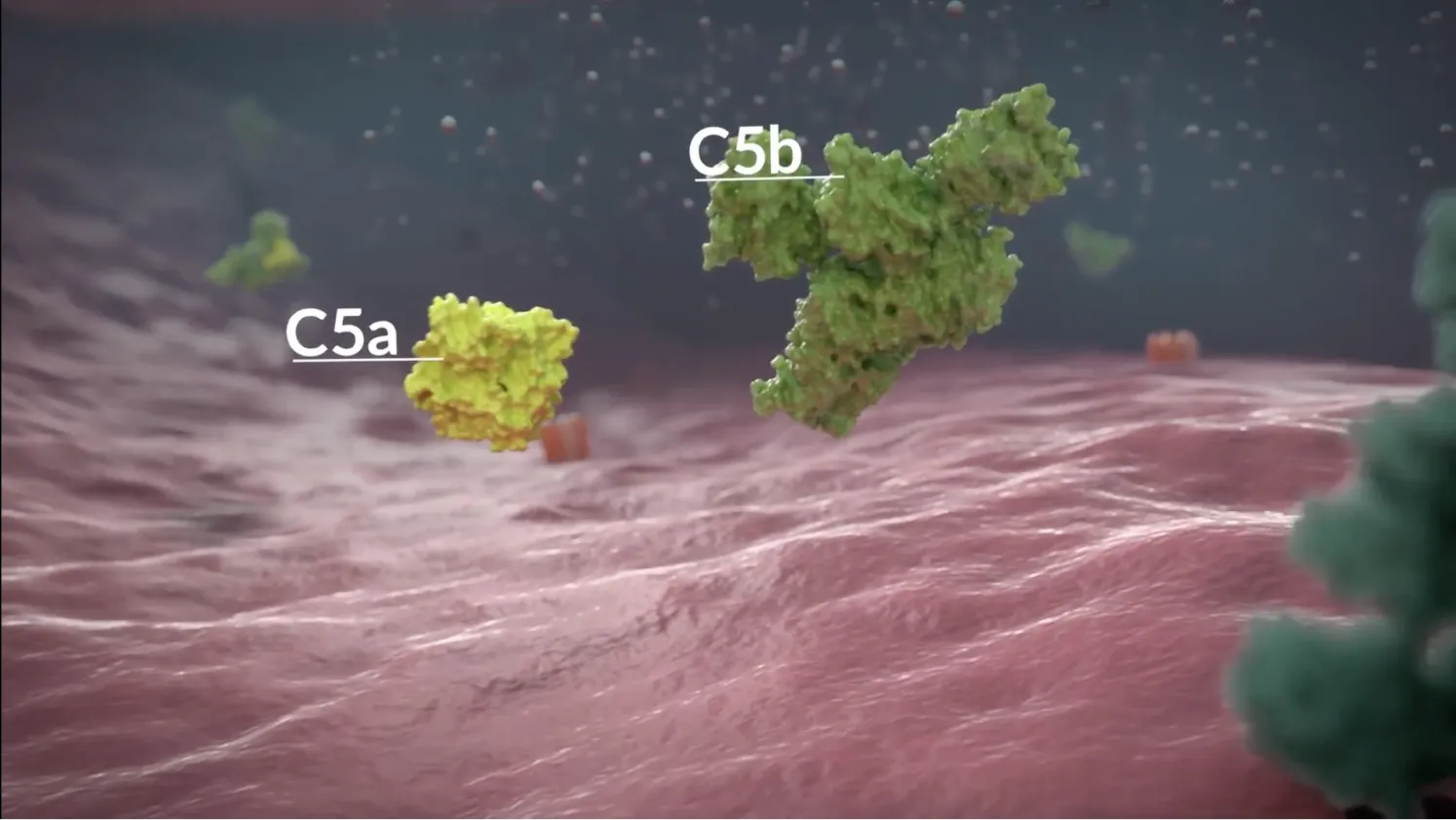
In anti-AChR antibody-positive gMG, complement activation leads to damage at the neuromuscular junction2,4




AChR, acetylcholine receptor; C1q, complement component 1q; C3, complement component 3; C5, complement component 5; C5a, complement component 5a; C5b-9, complement component 5b-9; gMG, generalised myasthenia gravis; MAC, membrane attack complex; NMJ, neuromuscular junction.
Healthcare professionals are asked to report any suspected adverse reactions. Please report any adverse reactions via your national reporting system. Adverse events should also be reported to Alexion pharmaceuticals by the following link: https://contactazmedical.astrazeneca.com/
