

Patients with gMG may face:3,5
Metabolic conditions
(weight gain, hyperglycaemia, diabetes)
Osteoporosis
Neuropsychiatric issues
Ophthalmologic conditions
Cardiovascular issues
(hypertension and arrhythmias) especially if pre-existing
Gastrointestinal issues
Electrolyte imbalances
Increased risk of infection
Myopathy

healthcare professional

REGAIN was a Phase 3, randomised, double-blind, placebo-controlled trial with an open-label extension (OLE). Patients were randomised to receive either SOLIRIS® (n = 62) or placebo (n = 63) for 26 weeks and allowed to enter the OLE period for up to 4 years.1,6,10,11
Outcome: 4.2-point improvement in mean MG-ADL total score from baseline to Week 26 among patients receiving SOLIRIS® vs 2.3-point improvement in patients receiving placebo (P = 0.006).1 In the study, the average baseline total score for people receiving SOLIRIS® was 10.5; for people receiving placebo, it was 9.9.1,6,10,11
REGAIN OLE was the long-term extension trial following the 26th week of REGAIN. Participants received open-label SOLIRIS® 1200 mg every 2 weeks for up to 4 years after a 4-week blinded induction period. At last OLE assessment, 88.0% (103/117) of participants were using 1 IST vs 98.3% (115/117) at OLE baseline.6
LIMITATION: Results and clinical outcomes should be interpreted with caution as the study was designed to evaluate safety and lacked a control group.6
LIMITATION: Results and clinical outcomes should be interpreted with caution as the study was designed to evaluate safety and lacked a control group.6
In the OLE, there were more patients who stopped or reduced their dose of an IST by the last assessment than those who started or increased their dose of an IST, with over 10% of patients stopping treatment with concomitant ISTs. Concomitant IST use was measured over a median of 32 months.6
Majority of patients reduced IST dose during REGAIN OLE:*6
* Reasons for patients stopping/reducing treatment include: symptom improvement, symptom worsening, new indication other than MG for IST use, side effects, and intolerance to existing IST. Others include temporary dosing/treatment changes in response to conditions such as asthma/chronic obstructive pulmonary disease, conjunctivitis, and urinary tract infections or to support surgery.6
Additional patient IST dose changes:
- Any given patient may have experienced multiple changes in IST use for a variety of reasons. 71/117 started or increased their dose of ≥ 1 IST, with 44/117 due to symptom worsening6
Concomitant ISTs included, but were not limited to: prednisone, prednisolone, methylprednisolone, methylprednisolone sodium succinate, meprednisone, azathioprine and mycophenolate mofetil.6
Corticosteroid use was reduced and/or stopped in 47.9% of patients during the REGAIN OLE6
Changes in patients’ corticosteroids (prednisone) reduced during REGAIN OLE:6
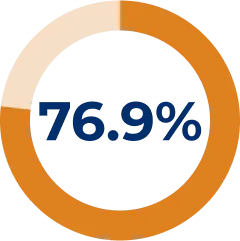
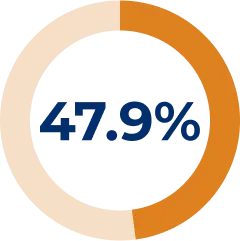
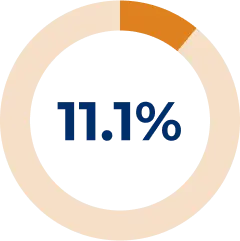
* Corticosteroids used in the OLE were prednisone, prednisolone, methylprednisolone, methylprednisolone sodium succinate, and meprednisone.6
† During the OLE, 4 additional patients used prednisone and related corticosteroids (90 patients at OLE baseline).6
† During the OLE, 4 additional patients used prednisone and related corticosteroids (90 patients at OLE baseline).6
Reductions in mean daily corticosteroid dose from OLE baseline to last assessment were observed among all patients and those who reduced and/or stopped corticosteroids6
At OLE baseline of all patients taking corticosteroids (N = 90):6
- Mean daily corticosteroid dose was 16 mg
At last assessment of all patients taking corticosteroids (N = 90):6
- Mean daily corticosteroid dose was 12 mg
- Mean daily dose reduced by 16.4% from baseline
Compared to baseline, some patients taking eculizumab were able to reduce their use of corticosteroids6
ELEVATE was a retrospective observational study using physician-reported EMR data. With physician-reported electronic medical records data, each patient (n = 119) served as their own control. ELEVATE retrospectively observed patient outcomes before SOLIRIS® initiation for up to 2 years and patient outcomes after SOLIRIS® initiation for up to 2 years.7
LIMITATION: ELEVATE was a retrospective analysis; therefore, results and clinical outcomes should be interpreted with caution.7
LIMITATION: ELEVATE was a retrospective analysis; therefore, results and clinical outcomes should be interpreted with caution.7
Observation: Improvement in MG-ADL total scores was observed by 3 months (2.6-point improvement, P < 0.001) and was sustained through 24 months (3.2-point improvement, P < 0.001). Mean MG-ADL total score at baseline was 8.0. A 3.2-point reduction was observed in mean MG-ADL total score from baseline at Week 104 in patients taking SOLIRIS®.7
64% of patients demonstrated reduced prednisone dosage in the ELEVATE analysis7
Retrospective chart analysis of prednisone use over 24 months of SOLIRIS® treatment7
- At data cut-off, 82% of all patients in ELEVATE received SOLIRIS® for ≥ 12 months (n = 98)7
- At treatment initiation, 69 patients were receiving prednisone7
Percentage of patients with reduced corticosteroids (prednisone) in ELEVATE:7
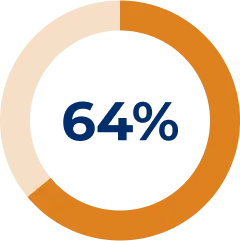

- The percentage of patients who did not change their dosage was 20%, while 3% of patients increased their prednisone dosage (median time: approx. 8 months)7
- These results support findings from the REGAIN trial and its open-label extension, and corroborate other real-world studies6,7
Data were collected from US patients enrolled in a global registry.14,15 Patients with gMG (n = 110) were treated with SOLIRIS® for ≥ 1 year and had data on concomitant therapy use from 12 months before SOLIRIS® initiation to data cut after ~ 3 years.15 48.7% of patients were taking corticosteroids, including prednisone, at SOLIRIS® initiation.4
LIMITATION: Observations should be interpreted with caution as this was a retrospective observational review from a registry.14,15
LIMITATION: Observations should be interpreted with caution as this was a retrospective observational review from a registry.14,15
Observation: The mean MG-ADL total score decreased 4.3 points (from 8.3 before SOLIRIS® treatment to 4.0 at or after registry enrolment).15
More patients with gMG were able to treat with ≤ 5 mg of prednisone compared to baseline in a global gMG registry14
Observations after 1 year of treatment with SOLIRIS®
At treatment initiation, the number of patients treated with a prednisone dose ≥ 10 mg/day was 31 (47%).14
Prednisone dosages reduced after 1 year of treatment in the registry:14
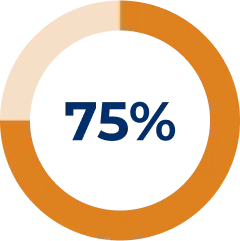
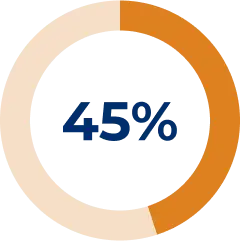
The objective of this analysis was to assess changes in concomitant therapies after initiation of SOLIRIS® in patients with gMG. Reasons for concomitant therapy discontinuation were not assessed.14
There were observed reductions in corticosteroid (prednisone) use to < 10 and < 5 mg per day with the use of SOLIRIS®.14
REGAIN OLE STUDY LIMITATION: Results and clinical outcomes should be interpreted with caution as the study was designed to evaluate safety and lacked a control group.6
To reduce the risk of infection, all patients must be vaccinated against meningococcal infections at least 2 weeks prior to initiating SOLIRIS® unless the risk of delaying SOLIRIS® therapy outweighs the risk of developing a meningococcal infection. Patients who initiate SOLIRIS® treatment less than 2 weeks after receiving a tetravalent meningococcal vaccine must receive treatment with appropriate prophylactic antibiotics until 2 weeks after vaccination. Patients must receive vaccination according to current national vaccination guidelines for vaccination use.1
Please consult the Summary of Product Characteristics prior to prescribing.
Please consult the Summary of Product Characteristics prior to prescribing.
The most common adverse reaction to SOLIRIS® was headache, (occurred mostly in the initial phase of dosing), and the most serious adverse reaction was meningococcal infection.1
AChR, anti-acetylcholine receptor; CI, confidence interval; EMR, electronic medical record; gMG, generalised myasthenia gravis; IST, immunosuppressive therapy; LS, least squares; MG-ADL, Myasthenia Gravis Activities of Daily Living; OLE, open-label extension; QMG, quantitative myasthenia gravis.
Adverse Event Reporting
Please report any adverse events via your national reporting system. Adverse events can also be reported to Alexion Pharmaceuticals by the following link: https://contactazmedical.astrazeneca.com/
Please report any adverse events via your national reporting system. Adverse events can also be reported to Alexion Pharmaceuticals by the following link: https://contactazmedical.astrazeneca.com/
