

Adapted from Howard JF, et al. Lancet Neurol. 2017;16(12):976–986.2
* In the worst-rank ANCOVA, patients with discontinuation, crisis, or rescue therapy were ranked lowest—regardless of response to SOLIRIS®—while all others were ranked according to response to treatment. Of the seven patients who discontinued, four met protocol criteria for clinical worsening (two in the placebo group and two in the SOLIRIS® group). The other three, all of whom received SOLIRIS®, discontinued the study because of adverse events that did not reflect worsening of myasthenia gravis and did not receive rescue therapy, and all three had a clinically meaningful benefit in response to treatment. The adverse effects that led to discontinuation were Moraxella lacunata bacteraemia, bowel perforation, and adenocarcinoma of prostate gland. At the time of discontinuation, these three patients had 3, 7, and 7-point improvements in MG-ADL, respectively.2- The primary endpoint was the change from baseline in the MG Activities of Daily Living profile (MG-ADL – a patient reported outcome measure validated in gMG) total score at week 261
- The primary analysis of the MG-ADL was a worst-rank ANCOVA with a mean rank of 56.6 for SOLIRIS® and 68.3 for placebo, based on 125 study patients (P = 0.0698)1
Adapted from Howard JF, et al. Lancet Neurol. 2017;16(12):976–986.2
* The proportion of clinical responders at Week 26 with no rescue therapy was 59.7% on SOLIRIS® compared with 39.7% on placebo (P = 0.0229).1 A clinical responder in the MG-ADL total score was defined as having at least a 3-point improvement; of note, this threshold exceeds the change considered to be clinically meaningful, that is, a 2-point reduction in the MG-ADL score and is therefore a more rigorous criteria.2
†, ‡ and § represent the 2-sided nominal P-value for the comparison between SOLIRIS® and placebo using a CMH test. † P <0.05; ‡ P < 0.01; § P < 0.001.2

healthcare professional
Adapted from Muppidi S, et al. Muscle Nerve. 2019;14–24.3
* REGAIN open-label study limitation: Observations in the open-label extension (OLE) study are based on an interim analysis with a primary goal of evaluating safety. Any inference of efficacy or clinical significance should be interpreted with caution since the study was open-label and lacked control group.3Adapted from Howard JF, et al. Ann Clin Transl Neurol. 2021;8(7):1398–1407.4
* Responses were defined as improvement in MG-ADL score, ≥ 3 points, or QMG score, ≥ 5 points.4Discontinuation of SOLIRIS® should be considered in a patient who shows no evidence of therapeutic benefit by 12 weeks.3 Use of SOLIRIS® in refractory gMG treatment has only been studied in the setting of chronic administration.1
Patients who discontinue SOLIRIS® treatment should be carefully monitored for signs and symptoms of disease exacerbation.1
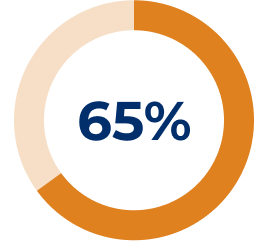
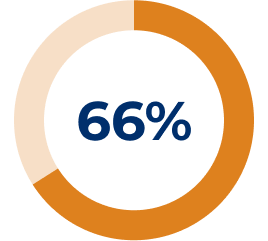
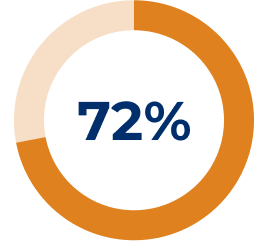
Adapted from Muppidi S, et al. Muscle Nerve. 2019;14–24.3
* All reported exacerbations.3
† Model-based even rate per 100 patient-years is based on a generalised estimating equation Poisson regression repeated-measures model with the number of events as the dependent variable, the logarithm of patients-years as the offset variable, and the study phase indicator (pre-study, placebo or SOLIRIS®) as the factors assuming a compound symmetry correlation structure.3
‡ Compared to REGAIN placebo group.3
§ Exacerbation events requiring rescue therapy.3