

To reduce the risk of infection, all patients must be vaccinated against meningococcal infections at least 2 weeks prior to initiating SOLIRIS® unless the risk of delaying SOLIRIS® therapy outweighs the risk of developing a meningococcal infection. Patients who initiate SOLIRIS® treatment less than 2 weeks after receiving a tetravalent meningococcal vaccine must receive treatment with appropriate prophylactic antibiotics until 2 weeks after vaccination. Patients must receive vaccination according to current national vaccination guidelines for vaccination use.3
Please consult the Summary of Product Characteristics prior to prescribing.

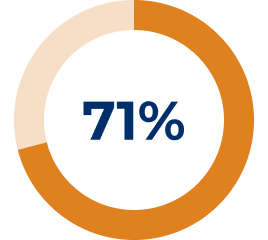
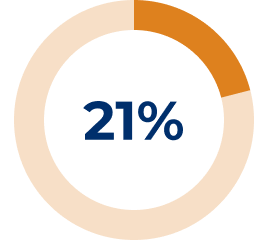
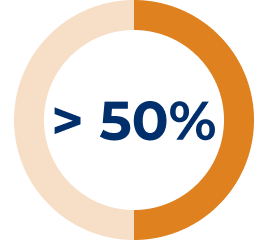
* In the REGAIN study, refractory gMG patients were defined as: 1) Patients who failed treatment for at least one year with 2 or more immunosuppressant therapies (either in combination or as monotherapy), i.e., patients continued to have impairment in activities of daily living despite immunosuppressant therapies. OR 2) Patients who have failed at least one immunosuppressant therapy and required chronic plasma exchange or IVIg to control symptoms, i.e., patients require PLEX or IVIg on a regular basis for the management of muscle weakness at least every 3 months over previous 12 months.3
† ICU admission: at Year 2: OR = 3.498 (1.514, 8.081) P = 0.0034; feeding tube use: At Year 2: OR = 84.639; (3.328, 2152.581); P = 0.0072.8

healthcare professional

