


healthcare professional
Please refer to the KOSELUGO® SmPC for full prescribing information


*There are special safety considerations for the use of KOSELUGO®, please refer to the SmPC for full safety information.
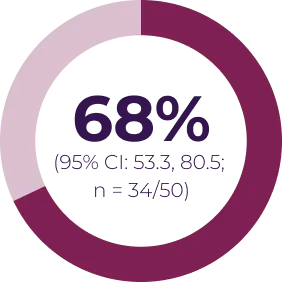
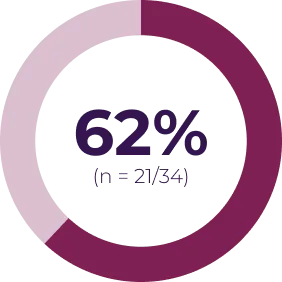
Most adverse events can be managed by dose interruption, reduction, or discontinuation. In Phase I of the SPRINT trial (N = 24), 13 patients required dose reduction for toxicity, and 3 patients discontinued treatment due to an adverse event possibly related to treatment. In SPRINT Phase II Stratum 1 (N = 50), 16 patients had ≥ 1 dose reduction and 5 patients discontinued treatment due to an adverse event possibly related to treatment.4
KOSELUGO® is a selective inhibitor of mitogen-activated protein kinase kinases 1 and 2 (MEK 1/2). KOSELUGO® blocks MEK activity and the rat sarcoma (RAS)- rapidly accelerated fibrosarcoma (RAF)-MEK- extracellular signal-regulated kinase (ERK) pathway. Therefore MEK inhibition can block the proliferation and survival of tumour cells in which the RAS-RAF-MEK-ERK pathway is activated.1,5

CI, confidence interval; ERK, extracellular signal-regulated kinase; GDP, guanosine diphosphate; GTP, guanosine triphosphate; MEK, mitogen-activated protein kinase kinase; NF1, neurofibromatosis type 1; PN, plexiform neurofibroma; RAF, rapidly accelerated fibrosarcoma; RAS, rat sarcoma viral oncogene homologue.
▼ This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information.
Please report any adverse events via your national reporting system. Adverse events should also be reported to AstraZeneca by visiting https://contactazmedical.astrazeneca.com or by calling 0800 783 0033.