


healthcare professional
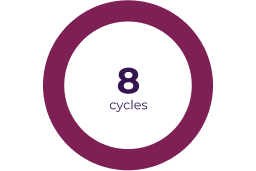
The efficacy and safety of KOSELUGO® was evaluated in SPRINT Phase II Stratum 1, an open-label, multicentre, single-arm study, conducted on 50 paediatric patients with neurofibromatosis type 1 (NF1) and inoperable plexiform neurofibromas (PN) that caused significant morbidity.1,2
The SPRINT study is ongoing. Efficacy data are presented based on a data-cut-off of March 2021.1
Key inclusion criteria:3
- Aged 2–18 years*
- NF1 and at least one inoperable, symptomatic measurable PN
- Inoperable PN was defined as PN that could not be completely removed surgically without risk for substantial morbidity due to encasement of, or close proximity to, vital structures, invasiveness or high vascularity of the PN
- Measurable PN was defined as a lesion of at least 3 cm measured in one dimension and suitable for volumetric magnetic resonance imaging
- ≥ 1 neurofibroma-related complication
- Able to swallow intact capsules
Key exclusion criteria:3
- Use of an investigational agent within the past 30 days
- Individuals who were pregnant or breastfeeding because of a potential risk of foetal and teratogenic adverse events
- Evidence of ongoing radiotherapy, chemotherapy, hormonal therapy directed at the tumour, immunotherapy or biologic therapy
- Clinically significant uncontrolled unrelated systemic illness
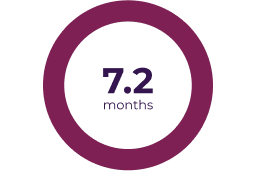
- 68% (n = 34/50; 95% CI: 53.3, 80.5) of patients achieved a confirmed partial response (reduction in PN volume by ≥ 20% compared with baseline, confirmed at a subsequent tumour assessment within 3–6 months)1
- No patients achieved a complete response1
Key secondary endpoints
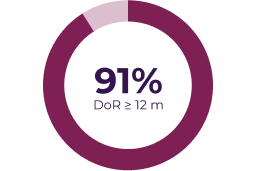
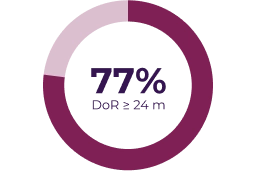
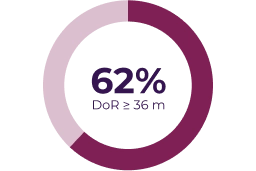

Adapted from: Gross AM et al. Neuro Oncol. 2023;25(10):1883–1894.5
Partial response was defined as > 20% tumor shrinkage from baseline (dashed line) and progressive disease was defined as > 20% increase from baseline or from best response if there was a prior partial response.5

▼ This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information.
Please report any adverse events via your national reporting system. Adverse events should also be reported to AstraZeneca by visiting https://contactazmedical.astrazeneca.com or by calling 0800 783 0033.
