


* Due to its mechanism of action, the use of ULTOMIRIS® increases the patient's susceptibility to meningococcal infection/sepsis (Neisseria meningitidis). To reduce this risk of infection, all patients must be vaccinated against meningococcal infections at least two weeks prior to initiating ULTOMIRIS®
† Stable–dose acetylcholinesterase inhibitors and ISTs (including corticosteroids) were permitted during the randomised controlled period. Dose changes were permitted in the open–label extension.2,3
AChR, acetylcholine receptor; gMG, generalised myasthenia gravis; IST, immunosuppressant therapy; OLE, open–label extension; SOC, standard of care.
ISTs, immunosuppressant therapies.

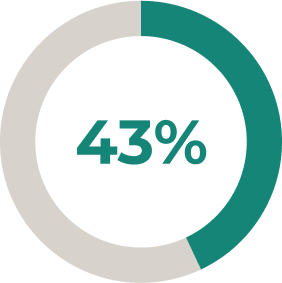
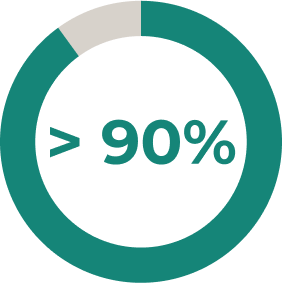
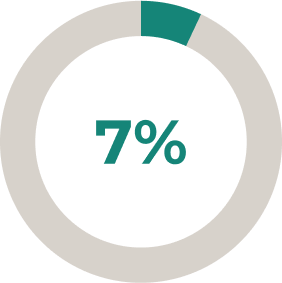
* Stable-dose acetylcholinesterase inhibitors and ISTs (including corticosteroids) were permitted during the randomised controlled period. Dose changes were permitted in the open-label extension2
ISTs, immunosuppressant therapies.AChR, acetylcholine receptor; gMG, generalised myasthenia gravis; IST, immunosuppressant therapy; IVIg, intravenous immunoglobulin; MG, myasthenia gravis;
MGFA, Myasthenia Gravis Foundation of America; PE, plasma exchange; SOC, standard of care.
Adverse Event Reporting
Please report any adverse reactions via your national reporting system. Adverse events should also be reported to Alexion pharmaceuticals by the following link: https://contactazmedical.astrazeneca.com

Adapted from: Vu T, et al. N Eng J Med Evid. 2022; 1(5).2
Improvements in MG-ADL were observed within 1 week of treatment, but the primary endpoint was at Week 26. Therefore, results should be interpreted with caution.2
CHAMPION-MG study limitations: Data shown are least-squares means and 95% confidence intervals (Cls), using a mixed model for repeated measures; 95% Cls were not adjusted for multiplicity.2
* Stable-dose acetylcholinesterase inhibitors and ISTs (including corticosteroids) were permitted during the randomised controlled period.2
BL, baseline; Cl, confidence interval; C5, complement component 5; IST, immunosuppressant therapy; LS, least squares; MG–ADL, Myasthenia Gravis–Activities of Daily Living; SD, standard deviation.
Adapted from: Vu T, et al. N Eng J Med Evid. 2022;1(5).2
* Stable-dose acetylcholinesterase inhibitors and ISTs (including corticosteroids) were permitted during the randomised controlled period.2
BL, baseline; Cl, confidence interval; IST, immunosuppressant therapy; LS, least squares; QMG, Quantitative Myasthenia Gravis; SD, standard deviation.
Adapted from: Meisel A, et al. [published online ahead of print]. J Neurol. 2023;1-14.3
* Patients were randomised to receive either ULTOMIRIS® (n=86) or placebo (n = 89) for 26 weeks and were subsequently allowed to enter the open–label extension (OLE) period for up to 4 years.2
† Stable–dose acetylcholinesterase inhibitors and ISTs (including corticosteroids) were permitted during the randomised controlled period.2
‡ Dose changes of concomitant acetylcholinesterase inhibitors and ISTs (including corticosteroids) were permitted during the open–label extension.3
§ This was an open–label extension study and lacked a control group.3
BL, baseline; Cl, confidence interval; IST, immunosuppressant therapy; LS, least squares; MG–ADL, Myasthenia Gravis Activities of Daily Living; OLE, open–label extension; SD, standard deviation.In the OLE period, 28% of patients decreased their
daily dose of corticosteroids, with 6% discontinuing
corticosteroids altogether*†3
* N = 161, 45 patients decreased their corticosteroid dose and 10 patients discontinuing their corticosteroids entirely.3
† In patients followed for 60 weeks in the open–label extension period.3
Adapted from: Meisel A, et al. [published online ahead of print]. J Neurol. 2023;1-14.3
* Patients were randomised to receive either ULTOMIRIS® (n=86) or placebo (n = 89) for 26 weeks and were subsequently allowed to enter the open–label extension (OLE) period for up to 4 years.2
† Stable–dose acetylcholinesterase inhibitors and ISTs (including corticosteroids) were permitted during the randomised controlled period.2
‡ Dose changes of concomitant acetylcholinesterase inhibitors and ISTs (including corticosteroids) were permitted during the open–label extension.3
§ This was an open–label extension study and lacked a control group.3
BL, baseline; Cl, confidence interval; IST, immunosuppressant therapy; LS, least squares; MG–ADL, Myasthenia Gravis Activities of Daily Living; OLE, open–label extension; SD, standard deviation.ULTOMIRIS®: a greater proportion of patients achieved clinically meaningful improvements in QMG total score vs placebo*1,2
30% (n = 27/76) of patients taking ULTOMIRIS® had a ≥ 5–point improvement in QMG total score vs 11.3% (n = 10/78) taking placebo (P = 0.005).2
Adapted from: Vu T, et al. N Eng J Med Evid. 2022; 1(5).2
* Stable-dose acetylcholinesterase inhibitors and ISTs (including corticosteroids) were permitted during the randomised controlled period.2
IST, immunosuppressant therapy; QMG, Quantitative Myasthenia Gravis- Change from baseline to Week 26 in revised 15–Component MG–QoL15r: –3.3 for ULTOMIRIS® and –1.6 for placebo
- Change from baseline to Week 26 in the Neurological Quality of Life Fatigue. score: –7.0 for ULTOMIRIS® and –4.8 for placebo
- Neither of these endpoints were statistically significant
- More patients taking ULTOMIRIS® achieved a ≥ 3–point improvement in MG–ADL total score vs placebo
- 57% (n= 47/78) of patients taking ULTOMIRIS® had a ≥ 3–point improvement in MG–ADL total score vs 34% (n = 30/82) of patients taking placebo, this was not statistically significant
- Change from baseline to Week 26 in the Myasthenia Gravis Activities of Daily Living (MG–ADL) total score*
- Change from baseline to Week 26 in the Quantitative Myasthenia Gravis (QMG) total score§1d
- The proportion of patients with improvements of at least 5 points in their QMG total score1
- Change in the revised Myasthenia Gravis Quality of Life 15–ltem (MG–QoL15r)2
- Change in Neurological Quality of Life (Neuro–QoL) Fatigue assessment2
- The proportion of patients with improvements of at least 3 points in their MG–ADL total score1
† Hierarchical testing proceeded from the first to the fifth endpoint, and if statistical significance was not achieved (P–value > 0.05), then subsequent endpoints were not considered statistically significant.2
‡ All secondary endpoints are at Week 26.1
§ The QMG is a 13–item categorical scale assessing muscle weakness. Each item is assessed on a 4–point scale where a score of 0 represents no weakness and a score of 3 represents severe weakness. The total score ranges from 0 to 39, where higher scores indicate more severe impairment.2
gMG, generalised myasthenia gravis; IST, immunosuppressant therapy; MG–ADL, Myasthenia Gravis–Activities of Daily Living; MGFA, Myasthenia Gravis Foundation of America; MG–QoL15r, the revised Myasthenia Gravis Quality of Life 15–ltem; Neuro–QoL, Neurological Quality of Life; QMG, Quantitative Myasthenia Gravis.
Patients reaching minimal manifestation status may
be better able to perform everyday activities.4
BL, baseline; Cl, confidence interval; C5, complement component 5; IST, immunosuppressant therapy; LS, least squares; MG–ADL, Myasthenia Gravis Activities of Daily Living; MG–QoL15r, revised Myasthenia Gravis Quality of Life 15–item; OLE, open–label extension; SD, standard deviation; QMG, Quantitative Myasthenia Gravis.
Adverse Event Reporting
Please report any adverse reactions via your national reporting system. Adverse events should also be reported to Alexion pharmaceuticals by the following link: https://contactazmedical.astrazeneca.com

- The most serious adverse reactions are meningococcal infection (0.7%), including meningococcal sepsis, encephalitis meningococcal, meningococcal infection and disseminated gonococcal infection (0.1%).1
- The most common adverse reactions with ravulizumab are headache (28.2%), upper respiratory tract infection (19.9%), nasopharyngitis (19.5%), diarrhoea (16.9%), pyrexia (16.4%), nausea (13.7%), arthralgia (13.2%), fatigue (13.1%), back pain (12.6%), abdominal pain (11.8%), and dizziness (10.1%).1
* In generalised myasthenia gravis (gMG), paroxysmal nocturnal haemoglobinuria (PNH), atypical haemolytic uraemic syndrome (aHUS) and aquaporin–4 antibody–positive neuromyelitis optica spectrum disorder (AQP4 Ab+ NMOSD).1
- Serious adverse events were reported in 20 (23%) patients with gMG receiving ULTOMIRIS® and in 14 (16%) patients receiving placebo2
- The most frequent serious adverse events were related to worsening of gMG (1 patient receiving ULTOMIRIS® and 3 patients receiving placebo) and COVID–19 (2 receiving ULTOMIRIS® and 1 receiving placebo)2
The most serious adverse reactions are meningococcal infection (0.7%), including meningococcal sepsis, encephalitis meningococcal, meningococcal infection and disseminated gonococcal infection (0.1%).1
Across all indications, the most common adverse reactions with ravulizumab are headache (28.2%), upper respiratory tract infection (19.9%), nasopharyngitis (19.5%), diarrhoea (16.9%), pyrexia (16.4%), nausea (13.7%), arthralgia (13.2%), fatigue (13.1%), back pain (12.6%), abdominal pain (11.8%), and dizziness (10.1%).1
AChR, acetylcholine receptor; aHUS, atypical haemolytic uraemic syndrome; AQP4 Ab+ NMOSD, aquaporin–4 antibody–positive neuromyelitis optica spectrum disorder; C5, complement component 5; gMG, generalised myasthenia gravis; PNH, paroxysmal nocturnal haemoglobinuria.
Adverse Event Reporting
Please report any adverse reactions via your national reporting system. Adverse events should also be reported to Alexion pharmaceuticals by the following link: https://contactazmedical.astrazeneca.com

healthcare professional
